|
|
Summary
The genus Rattus emerged from the Murid family about 3.5 million years ago, and Norway rats themselves emerged about 2 million years ago. Norway rats originated in what is now northern China. Norway rats traveled to Europe in human ships in the 16th century and reached the New World in the 18th century. Today, Norway rats live in human cities, suburbs, and agricultural areas in a human-dependent relationship called commensalism (for more, see History of the Norway rat)
Wild rats live in colonies. Female rats, usually related to each other, live in little groups of one to six in a little burrow system of their own. They each have their own nest chamber, but they may share the burrow and may raise their young together (called communal nesting). When the offspring are weaned, the young males disperse.
The organization of male rats and the rat mating system changes depending on the population density of the colony. At low densities, a male rat monopolizes a burrow of females. He defends a territory, keeping other males away from the burrow and the surrounding area, and he mates only with the females of his group. One male mating with multiple females is a polygynous mating system. At low densities, Norway rats are therefore territorial and polygynous.
At high densities, males can no longer defend a burrow against intruders. There are simply too many intruders. At high densities, the social system becomes despotic, with one male becoming socially dominant while others become socially subordinate. Males no longer defend female burrows. Instead, when a female comes into heat a group of males rushes her and mates with her sequentially, with little or no competition between themselves. Males may mate with multiple estrus females this way, and females mate with multiple males. This mating system is polygynandrous. At high densities, Norway rats are therefore despotic and polygynandrous.
Wild rat aggressive behavior is similar to that seen in domestic rats, with fighting, chasing, biting, and boxing. However, wild rats don't display some agonistic behaviors seen in domestic rats, such as the sidling posture and the belly-up posture. The difference is probably due to the restricted environment of the cage, which leads to prolonged, escalated conflicts and the necessity of protracted self-defense in close quarters.
Rats don't live long in the wild -- the average lifespan is probably less than a year. In one study, the researcher found that 95% of rats living at a farm were no longer alive a year later. So rats suffer very high mortality in the wild. However, their birth rate is high as well, ranging from 1% to 6% of the carrying capacity per month during population recovery after an artificial reduction.
The natural habitat of the wild Norway rat
What is the natural habitat of the wild Norway rat? Thousands of years ago, the rat lived as a wild rodent in northern China (for more, see History of the Norway rat ). That is where they originated, but little is known about their ecology and habits there.
At some point, however, rats in China threw their lot in with human beings. Rats moved in to the early human settlements: they invaded human homes and farms, barns and buildings and villages. This relationship probably benefitted the rats because humans unwittingly provided them with food in the form of scraps and garbage. Humans provided warmth and shelter in and under their homes, and humans kept the rat's natural predators away. So the rat came to live with us in a human-dependent relationship called commensalism.
When humans traveled rats came along as stowaways. Black rats came with us on trade routes and ships in the Middle Ages, and Norway rats several centuries later, spreading from East to West. They traveled to the New World in sailing ships. Wherever humans went, our rat parasites went too.
Today, rats are found just about everywhere humans are found. They live in cities and farms and subways, in attics and basements, on oil tankers and barges, all over the world. Except in northern China, rats are not native wildlife. They emigrated with humans and settled in foreign lands, living off their human hosts. This stowaway strategy has worked well for the little rodent from China. Rats now cover the globe, in a population explosion that spans the entire earth.
So, what is the rat's natural habitat? Today the natural habitat of the wild Norway rats is wherever we are. Human settlements are now the ecological niche of the wild rat: the farm, the city, the garbage dump, the sewer, the subway.
Occasionally, when the climate is mild, rats may successfully establish themselves and flourish as independent wild animals separate from their human hosts. They may live in grainfields during the summer, they may successfully colonize small tropical islands. But the vast majority of wild rats prefer to live where the going is good, the food is free, and the predators are scarce: in granaries, garbage dumps, back alleys, farms, and your attic.
Wild Norway rat burrows
|
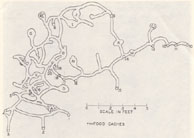
Elaborate Norway rat burrow system. From Calhoun 1963. Click for a larger image. |
Burrows are not unchanging entities. Burrows grow, change, and fall into disrepair as their inhabitants dig new tunnels, fill in old ones, or stop maintaining certain sections. In fact, the structure of a burrow system is related to the social relationships of the rats who dig and maintain it. If the social structure of the group breaks down, the rats are no longer able to maintain a complex burrow system and it falls into disrepair.
Parts of a burrow
- Entrance: Usually placed in a sheltered and/or sloped location. May be sealed with cut grass or dirt.
- Tunnel: Straight tunnel segments have a median width of 8.3 cm. This width allows only one rat to pass at a time. Straight tunnel segments have a median length of 29.8 cm. After that distance, a tunnel usually bends, ends in a nest cavity, splits into two tunnels, or dead-ends.
- Cavity: small chamber of varying size, median 18.5 x 22.1 cm, which could accomodate 7 rats. The smallest
chambers could accomodate only 3 rats, the largest 11 rats. Chambers can have several uses:
- nest cavity: contains bedding material, used for sleeping and rearing young litters.
- food-cache: used for food storage
- Nest: built in a nest cavity, it consists of shredded material and serves as a sleeping place. There are
three kinds of nest:
- pad: simplest kind of nest. Consists of just a few flat objects (leaves, bits of paper) which elevate one rat above the floor.
- "cup-shaped" nest: larger nest may with a cup shape. Made with finer texture grass or shredded paper interwoven a bit to form the walls of the cup. The cup is lined with flat objects.
- Hooded nest: Organized nest in which the walls grow so high they form a ceiling, and the nest becomes a hollow sphere with just one opening. These nests are sometimes built by mothers for litters, but tend to be rather rare.
Typical features of a burrow system (average of 44 burrow systems)
- tunnel segments: 16
- exits: 6.8
- chambers: 4.5
- adult rats: 5.5
Growth of a burrow system: Burrows are frequently started by a pregnant rat just before giving birth. Rats like to start burrows against vertical surfaces(e.g. a man-made wall), under flat survaces (e.g. under a board placed on the ground), under overhead cover (e.g. shrub, overhanging concrete, raised man-made floors), on slopes, and near sources of food and water. Dirt excavated from the initial tunnel is usually piled around the tunnel entrance in a burrow mound.
The initial tunnel usually ends in a nest cavity. After a few days, a second entrance is added by digging from below. This second hole has no excavated dirt around it and is called a bolt hole, and may serve as escape exits in case the burrow is invaded. Later expansions of the burrow system follows this same pattern of tunnel/cavity/bolt hole. The second cavity often becomes a food-cache, and is added by the mother rat shortly before the young are weaned. This allows the young to start on solid food in the safety of their home burrow.
Once established, these simple burrows serve as centers for the elaboration of extensive burrow systems.
Modifying the burrow: Rats prefer a certain distance between themselves and the ceiling of the nest cavity. As the rat brings in nesting material, the rat finds itself closer to the ceiling than it likes, so it removes dirt from the roof and carries it out. Over time, the bedding gets soiled and the rat brings more bedding in, and the process is repeated. Eventually, the nest cavity approaches the surface and its thin ceiling collapses.
Rats seal entrances temporarily with cut vegetation. These seals are easy to remove or squeeze through. Lactating mothers, especially those who have just given birth, may seal burrows in this way.
Rats also permanently seal entrances with loose dirt and lumps of dirt pushed up from below. they are packed into place with the forefeet. They may also carry dirt out another entrance and pack it in to the neighboring one which is being sealed. A rats may seal a burrow when it feels a draft and when it is of low social rank. The seal of a low ranking rat may prevent higher ranking rats from coming in and harassing it. The burrows of low-ranking rats also tend to leak more, so such seals may help counteract such leaks.
Trail systems: outside the burrow, rats tend to confine their movements to the same routes every day. Gradually, trails form on the surface.
- Reference: Calhoun (1963)
- See also: Boice (1977)
How do Norway rats behave in the wild?
The Norway rat (Rattus norvegicus) live in colonies that can number in the hundreds. There are two social systems for females and males.
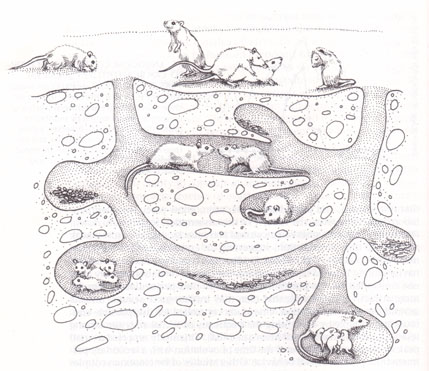
Female social system: Wild females may raise young alone (Telle 1966, as reported in Schultz and Lore 1993), or up to six reproductive females may share a ground burrow, each with a separate nest chamber. The females are often related through the mother's side and they may collectively nurture their young (Steineger 1950, as reported in Schultz and Lore 1993; Moore 1999, summarizing Calhoun 1963 and Barnett 1975) (for more, see article on communal nesting).
A burrow of females may or may not have one or more males associated with it and attempting, with variable success (depending on the density), to restrict mating access of other males to resident females (Moore 1999). A unit of several adult females, a few adults males, and their many subadult offspring are called breeding demes. Females may undergo estrus simultaneously.
Male social system: The social system of males is territorial or despotic depending on the population density (number of rats per square meter).
- At low population densities, male rats are territorial, defending territories around the burrow of one or more females and mating exclusively with those females (Barnett 1958). In a system of territorial males, each male has a separate home range. Relationships between males are determined by their spatial separation. In other words, a rat in a territorial social system is stimulated to aggress or yield to another rat according to its own spatial location and the location of the other rat. So, a rat on his own territory would attack an intruder, but if that same rat ventured onto another rat's territory he would yield to the resident male's attack (Lott 1984).
- At high population densities, (e.g. 1 rat per 5 square meters) territories become too costly to maintain due to intruder pressure, and the social system becomes a despotism. In a despotism, individuals have overlapping home ranges and their relationships are determined through social interactions independent of location. Rats in a despotic social system are stimulated to attack or yield by what a particular known individual does, rather than where he is (Barnett 1958, Lott 1984). In a despotic system, one rat becomes socially dominant and other rats become socially subordinate. Most pet and lab rats are kept at high densities, hence, domestic male rats tend to be socially despotic rather than territorial.
|
|
|
The typical social organization of wild Norway rats is a breeding deme. A breeding deme is a small group of animals consisting of a few females, a small number of males, and their many subadult offspring. From McClintock 1987. |
Mating system
Rat mating systems are a facet of social systems, and change with population density.
At low densities the mating system is mostly polygynous -- one male mates with multiple females. In this case, dominant males tend to monopolize an estrus female or a burrow of females.
At high densities such monopolies become difficult to maintain and the mating system becomes more polygynandrous -- multiple males mate with multiple females, through group mating and rushing. In this case large groups of males copulate sequentially with an estrus female, showing little if any overt competition between themselves (Moore 1999).
Polygynandrous mating leads to sperm competition within the female's reproductive tract, with sperm from many males competing to fertilize the female's eggs. The male that deposits more sperm has a higher chance of siring offspring, and males with larger testicles produce more sperm (Møller 1989). Over evolutionary time, polygynandrous mating gives rise to the rat's large testicles.
Group mating is stressful for the female, and intervals between copulations are brief (less than 1 minute), making conceptions less likely (Matthews and Adler 1977, Moore 1999). Such reproductive failure may be responsible for reducing the population growth rate and ultimately limiting the overall size of a population (see Nowak 1991). Under conditions in which a female can escape from the males, she hides after copulating and returns to mate after about 3 minutes (McClintock and Adler 1978).
It is not unusual for the social systems and mating systems of a species to change with population density. Social and mating systems may also change with resource distribution, spatial qualities of the landscape, predation levels, and so forth. For more on variation in vertebrate social systems, see Lott 1984.
|
Study of a low-density rat population that grows to high density: Calhoun (1963) (as reported in Nowak 1991) caught wild rats and placed them in a 0.1 hectare (1000 square meter) enclosure. He found that some males established individual territories in one part of the enclosure around burrows containing several females. Each male excluded the other males and mated only with the resident females. Reproduction occurred regularly and successfully. As the young matured, the young males were forced into another part of the enclosure. The population gradually grew larger. The low ranking males did not establish territories of their own, but formed large packs. When a female entered estrus, she would be followed by numerous males and mounted several hundred times a night. These stressful conditions resulted in poor reproduction, and the population stabilized at a high density of about 200 rats in the enclosure, or 0.2 rats per square meter. |
Study of a high-density rat population: The landfill population studied by Robitaille and Bovet (1976)
was a relatively high density one, with 0.10 to 0.17 rats per square meter. They observed mostly polygynandry
with a few instances of polygyny. They found that most mating was in the form of a group of males (from two to fifteen) rushing an estrus
female. One male mounted the female while the others crowded tightly around them. The mount was usually
interrupted by the female arching her back and jumping forward or turning to box with the dismounting male. She
also boxed to push back the group of males. Sometimes she withdrew into her burrow, and the males would mill
about at the entrance until she reappeared, whereupon the sequence was repeated. This activity could go on for
several hours (longest observed was 4 hours and 25 minutes). If only two males were involved, their interaction (e.g. boxing) gave the female a break. If more than two
males were present, however, an interaction between any two of them gave the other males a greater chance of
mating with the female. When this happened, the two fighting males quickly stopped and resumed following the
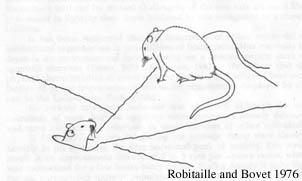
female. The only way for the female to escape, in this case, was to go down her burrow. In a few cases, a dominant male may achieve more than a brief monopoly over an estrus female. Some of the
largest dominant males may be able to maintain an exclusive monopoly over an estrus female by sequestering her
in a burrow and chasing the other males from the area (Figure 1). Almost all mating behavior occurred between 30 minutes and 4 hours after sunset, with a peak between 60 and
120 minutes after sunset.
High-density male-male competition: Socially dominant males within the male mating group may still have an edge over their peers because dominant males (studied in captivity) achieve more ejaculations and continue mating with individual females longer than other males in the group, thereby increasing the length of postcopulatory intervals following their ejaculations; both factors contribute to fertilization suggesting that male rank is correlated with reproductive success (Thor & Carr, 1979, after Moore 1999).
A female can bear a litter sired by multiple males, i.e. the resulting offspring are half-siblings, with the same mother but different fathers. So the male who can keep other males away from her right after he copulates with her increases the chance that more of her eggs will be fertilized by his sperm, thus increasing the chance that more of the ensuing litter will be his (Lanier et al. 1979). This gets into the areas of sperm competition and cryptic female choice, both hot topics in animal behavior right now (see Birkhead and Parker 1997 for more).
The female may benefit from mating with multiple males because males are less likely to commit infanticide after copulating with a female. Therefore, by mating widely, she may inhibit many males from killing her young.
|
|
Figure 1. Sideways posture of a dominant male sequestering an estrus female in her burrow (Robitaille and Bovet 1976). |
Agonistic behavior
Social dominance relationships among males in high density populations are established at about puberty through fighting and tend to be stable thereafter. Dominant males tend to be larger as adults, and hierarchies among subordinate males are indistinct. Mortality rates are higher for subordinates of both sexes, apparently involving adrenal hyperplasia (a reaction to stress) in addition to wounding or resource exclusion (Moore 1999). Social encounters are common; Robitaille and Bovet (1976), who studied rats in a landfill in Quebec, noted that screaming was continuous at their study site.
Agonistic behavior includes (Robitaille and Bovet 1976):
- heedfulness: rats stand motionless staring at each other, may be a challenge.
- threat displays (these are rare, may occur during heedfulness):
- teeth gnashing
- hair bristling
- back arching
- foot-drumming
- backward earth-throwing with the hind feet
- advance then retreat backwards a few steps
- attack: swift run toward another rat
- boxing: rats rear up and push each other with their forepaws
- jump fight: both rats jump toward each other with their four limbs thrown forward
- wrestling: rats grasp each other and roll rapidly around on the ground, frequently screaming
- retreat: withdrawal of loser. Includes:
- flight: loser runs away with no further participation by the winner
- pursuit: winner chases the loser
- escape jump: forward jump by loser to exit from a wrestling ball, or when touched on the rump by the pursuer
Robitaille and Bovet (1976) never saw sideways threat postures or belly-up rolls in wild rats. They also never observed amicable (friendly) behavior between wild adult rats: adult rats did not groom each other, nose each other, walk over or crawl under each other.
The end of agonistic encounters: Most agnonistic encounters (88%) ended in a retreat. During a retreat, the winner pursued the loser only about a third of the time (36%). Such pursuits were typically prolonged (over 20 meters in length), the loser ran in loops and circles with the winner just behind him or within 3 meters of him. Frequently, the pursuer reached the pursued rat and bit his rump. Sometimes the pursuer was cornered and then a jump fight or wrestle occurred until escape was possible. Most pursuits (81%) ultimately ended when the loser outran the winner. Other endings include: winner loses sight of loser, winner attacks another rat, or loser disappears down a burrow and winner is reluctant to follow.
The rest of the agonistic encounters (64%) the loser fled and was not pursued, in which case the loser noticed quickly, stopped within 2 or 3 meters of the winner, and disappeared into shelter.
Note: Because pursuits are long and conspicuous, they are more likely to draw the observer's attention than a simple flight, so flight is probably under-represented in these observations. Its actual occurrence may therefore be higher than 64%.
Bite distribution: 61% of bites were on the rump, tail or anal region 24% on the hind legs, and 15% on the head or shoulders (Robitaille and Bovet 1976; see Blanchard et al. 1975, Blanchard et al 1977, Pellis and Pellis 1987 for more on bite distribution and rump targeting).
Wild rats' response to intruders
Telle (1966) found that wild rats attack introduced intruders. However, these attacks are not sustained and the intruder is rarely seriously injured because he runs away. Once he reaches the periphery of the colony's territory, pursuit stops, and he is ignored as long as he stays far enough away.
Aggression toward intruders therefore seems to function to exclude intruders from resources like food, shelter, and sexual access to females available to residents, and to chase away surplus animals into adjacent areas.
Agonistic behavior in wild vs. domestic rats
The agonistic behavior of wild rats differs from that of domestic rats, on which most of the research on rat agonistic behavior has been done (see separate article on rat aggression)
Specifically, Robitaille and Bovet (1976) found that wild rat agonistic encounters in the wild were briefer than domestic rat encounters in captivity. Also, some agonistic behavior patterns observed in domestic rats were not observed in their wild counterparts: the sideways threat posture (sidle) and belly-up postures were never seen in the wild.
Also, the most common ending of an agonistic encounter between wild rats was a retreat, especially flight. In contrast, losing domestic rats in caged resident-intruder pairs spent a great deal of their time in the belly-up posture or boxing, and very little time in flight (Blanchard et al. 1977).
The differences between the agonistic behavior of wild and domestic rats are most likely due to the restricted nature of the caged environment, which leads to longer, escalated conflicts and the necessity of protracted self-defense in close quarters. Wild-trapped rats in captive colonies show aggressive behavior toward intruders that is similar to that of domestic rats: chasing, sidling, on-top, boxing, and biting (Takahashi and Blanchard 1982).
Age and aggression: young wild rats are immune from attack
Robitaille and Bovet (1976) divided their population into age-classes, and examined the agonistic behavior of each class:
|
Age class |
Weight |
Relative Abundance |
|
Class 1 |
less than 50 grams (mice-sized) |
10% |
|
Class 2 |
50-150 grams |
30% |
|
Class 3 |
150-350 grams |
50% |
|
Class 4 |
over 350 grams |
10% |
- Class 1: The very young Class 1 rats were never seen fighting in earnest and appeared to be immune from agonistic attack by older rats, and no wounds were ever recorded on them. These tiny rats spent a great deal of their time playing with each other, pursuing, boxing and wrestling with each other. The finding that very young rats are immune from attack is corroborated in domestic rats by Thor and Flannelly (1976) who found that very young rats (21-40 days old) are not attacked by adults.
- Class 2: Older juveniles from Class 2 play-fought with each other but were also observed to engage in agonistic encounters, and sometimes displayed wounding.
- Class 3 rats were the most frequently involved in the highest intensity agonistic encounters, even when controlled for their greater abundance. Class 3 rats were twice as numerous as Class 2 rats, but Class 3 agonistic encounters were over ten times as numerous as Class 2 agonistic encounters.
- Class 4 rats were rarely involved in hostile encounters, perhaps due to their scarcity. Also, the smaller rats seemed to avoid them.
Most agonistic encounters occurred between rats of the same size. A few, however, involved a larger rat attacking a smaller one, and appeared to occur in defense of a burrow opening.
Calhoun (1963), who studied wild rats in a semi-natural enclosure, notes that juvenile wild rats under 50 days of age are never attacked by older rats. As the youngsters grow older they may be attacked by adults, but under the age of 86 days they are almost never bitten.
Muriod rodents are nocturnal and crepuscular (active at night and twilight). The frequency of agonistic encounters tracked this activity pattern: agonistic encounters occurred throughout the night, with two peaks in frequency. The first peak occurred between 60 and 90 minutes after sunset, and the second peak occurred between 90 and 60 minutes before sunrise.
Dispersal
Inter-colony relationships vary from tolerance to hostility. Dispersal (both within and between colonies) is male-biased, meaning that females tend to stay in their home colonies while males tend to leave and join other colonies. Levels of social organization appear more permeable than among most primates and membership in a burrow or colony is flexible (Moore 1999).
Population Dynamics
How long do wild rats live in the wild?
Wild rats have an average lifespan of about 1 year (Jackson 1982). Specifically, a wild rat population experiences about 95% mortality, which means that only 5% of rats remain alive after 12 months (Davis 1948, see also Brooks 1973). The longest-lived rat in the Davis (1948) study, which examined 1036 wild rats, lived to be 70 weeks old, e.g. 1 year and 4 months. In urban areas, the chances of surviving one year appear to be even less than 5% (Jackson 1982).
Note that this "average lifespan" does not represent extremes. Whitaker (1980) reports that some wild rats can reach age 3, but such individuals are very rare. The vast majority of wild rats don't make it to their first birthday.
How long do wild rats live in a protected, semi-natural environment?
Calhoun (1963) conducted a two-year study in which he kept wild rats in a 1/4 acre enclosure that was almost entirely predator-proof. He provisioned the rats with regular food and water. He started with ten wild rats and allowed the individuals to breed and die naturally.
In Calhoun's study, male and female survivorship curves were different. Half of the male rats had died by age 300 days (10 months), and 95% had died by about age 550 days (18.3 months). The oldest male lived to be about 610 days old (20.3 months).
Female rats suffered less mortality than males: 70% were still alive at 500 days (16.7 months). After this point, however, females died rapidly: only 50% lived to age 550 days (18.3 months), and 95% of females had died by age 650 days (21.7 months) (Figure 147, p. 245). The oldest female lived to be about 660 days old (22 months).
Population Growth and Recovery
Emlen et al. (1948) performed a study on the recovery of urban Norway rat populations after greater or lesser reduction in numbers. The rate of recovery depended on how severely the initial populations were reduced
- Undisturbed (zero reduction): Rat populations that were undisturbed showed irregular fluctuations in population numbers but no consistent trends.
- Moderate reduction (original population decreased by 50 to 90%): the rat population started recovering early and increased at rates of about 4% (range: 2 to 6%) of the original pre-reduction number per month.
- Severe reduction (original population decreased by over 90%): the rat population recovered at a lower rate, between 1 and 3%, until they reached 10% of their original numbers, whereupon the rate of increase accelerated.
- Eradication (100% reduction): Rat populations that were completely eradicated remained that way for two years. After that progress followed the regular course: slow at the beginning, gradually accelerating as the new population gained a foothold.
In general, populations only slightly reduced and those severely reduced recovered at slower rates than populations moderately reduced. This implies a logistic curve: rat populations increase slowly at first, then multiply rapidly, then slow again as the population reaches its carrying capacity. After the population reaches its carrying capacity, the population numbers fluctuate randomly around a constant level.