- Summary
- Will rats and mice mate naturally?
- Mixing rat eggs and mouse sperm
- Bypassing the egg's protective covering
- Replacing a mouse egg's nucleus with a rat nucleus
- Merging rat and mouse embryos
- Transplants: the secondary chimera
- Transgenic rats and mice
- Hybrid cell lines
Summary
A hybrid is an offspring of two different species. Are rat-mouse hybrids possible?
The short answer is no.
Rats and mice are not that closely related. Under normal circumstances, rats and mice are not attracted to each other and will not mate. Even if the sperm of one and the eggs of the other are mixed together artificially, fertilization will not occur: the eggs of one do not allow entry of the other's sperm.
These natural barriers can be overcome in a laboratory, at least at first. Such attempts typically result in a hybrid embryo which dies after a few cell divisions.
For example, the egg's protective covering can be stripped off, allowing the sperm of the other species access to the egg. Sperm can also be injected directly into the egg. When this is done, fertilization occurs. The resulting hybrid embryo is not viable, however. It typically goes through one cell division, resulting in a two-cell embryo, then it degenerates.
Another technique involves removing the nucleus (which contains most of the DNA) from a mouse egg and replacing it with a rat's nucleus. The resulting embryo has 100% rat nuclear DNA, enclosed in a mouse egg with mouse cell contents (cytoplasm). These nuclear transplant hybrids sometimes live to the 2-cell stage, but then they degenerate. The rat nucleus and the mouse cytoplasm are fundamentally incompatible.
Early stage embryos can also be merged, creating chimeras. Early stage embryos are just tiny clusters of a few undifferentiated cells. Chimeras are typically created by combining two early-stage embryos, producing a single merged embryo. The resulting individual is made up of two populations of cells, each descended from one of two fertilized ova. Chimeras have been attempted between species, including rats and mice. These were created by merging early stage rat and mouse embryos. The resulting chimeras developed normally in vitro, but when they were inserted into a hormonally primed host they were lost during or shortly after implantation.
In one case, however, using a different technique, a few of the chimeric rat-mouse embryos implanted successfully. They developed normally in vivo for about a week, before being removed and examined. The researchers found that the mouse cells gained the advantage: the mouse cells came to dominate the embryo and rat cells became rare.
Secondary chimeras are created when cells, organs, or tissue from one species are implanted into another. This procedure (called xenografting), is frequently and successfully performed in laboratories. For example, rat bone marrow can be injected into an immunosuppressed mouse, where it takes hold and produces white blood cells. Or a piece of rat skin could be grafted onto an immunosuppressed mouse. These mice with rat-tissue transplants have cells from both species, and therefore fit the definition of a chimera, but they are technically not hybrids.
Two additional techniques deserve mention: the creation of transgenic rats and rat-mouse hybrid cell lines.
Transgenic rats are rats that carry a gene from another species. The foreign gene is inserted into the fertilized rat egg, and the resulting rat carries the new gene in every one of its cells. Many types of transgenic animals have been produced, including rats that carry a mouse gene. The genome of these transgenic rats is almost 100% rat, of course, as they only have one or a few genes from other species.
Lastly, hybrid cell lines can be created in laboratories by fusing cells from two species and maintaining the resulting merged cells in a culture medium. Many such hybrid cell lines have been created, including rat-mouse hybrid cell lines. These cells will not, however, grow up to be a whole hybrid organism.
Will rats and mice mate naturally?
|
|
In virto fertilization: mixing rat eggs and mouse sperm
So, what if the whole mating barrier is bypassed, and rat sperm and mouse eggs (oocytes), or vice versa, are mixed together in a petri dish? Will the sperm of one species fertilize the egg of the other, creating a hybrid?
|
|
|
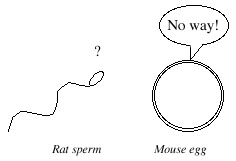
Figure 1. The eggs of one species refuse admittance to the sperm of the other. |
Only sperm from the same species can penetrate the protective covering to fertilize the egg. In rare cases, two
extremely closely related species may have "sperm keys" and "egg locks" that are so similar that the sperm key of
one sometimes manages to fit into the egg lock of the other, and cross-species fertilization occurs. For example,
horses and donkeys are just closely related enough to reproduce together, producing a mule (for more, see the hybridization page). But rats and mice are not closely related enough. So, rat and
mouse gametes mixed together in a petri dish just sit there: the eggs refuse admittance to the sperm and no
fertilization occurs (Fig. 1).
Bypassing the eggs's protective covering
What if the egg's zona pellucida, its protective covering, is bypassed? This can be done by (1) removal of the protective covering altogether, or (2) injection of a single sperm into the egg.
Removing the protective covering
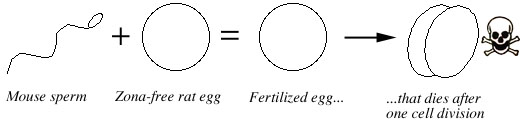
Rat eggs stripped of their zona pellucida can be fertilized by mouse sperm, but the reciprocal cross of rat sperm and zona-free mouse rarely results in fertilization (less than 5% success rate) (Hanada and Chang 1972).
Thadani (1980), found that mouse sperm successfully fertilized zona-free rat eggs and the resulting embryos went through the first few steps of normal development: they completed meiosis and a few of them went through their first cell division, thus forming a two-cell embryo. Then these two-cell embryos degenerated, though this could be because the culture mediums of the time were inadequate (Kishi et al. 1991) (Fig. 2).
|
|
|
Figure 2. A mouse sperm will fertilize a rat egg which has been stripped of its protective covering (the zona pellucida). The resulting hybrid embryo dies after one cell division, at the two-cell stage. The reciprocal cross (rat sperm + zona-free mouse egg) rarely results in fertilization. |
Injection of a single sperm into the egg
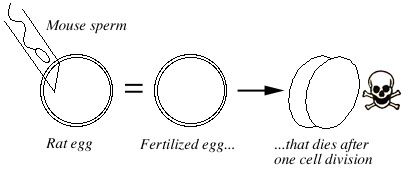
Thadani (1979, 1980) injected single mouse sperm into rat eggs (see also Witkowska 1982). As with the zona-free hybrid embryos, some of these embryos survived to the two-cell stage, then degenerated (Fig. 3).
The reciprocal cross, injecting a rat sperm into a mouse egg, does not work because mouse eggs are easily damaged by pricking (Market 1983, Mann 1988).
|
|
|
Figure 3. A mouse sperm injected into a rat egg will result in fertilization. The resulting hybrid embryo dies after one cell division, at the two cell stage. The reciprocal cross (rat sperm injected into mouse egg) doesn't work because mouse eggs are easily damaged by the needle. |
Nuclear transplant: replacing a mouse egg's nucleus with a rat egg's nucleus
In nuclear transplantation, the nucleus (the center part of the cell that contains most of the DNA) is scooped out of an unfertilized egg and replaced by the nucleus of another cell. The egg with its new complement of DNA is then activated artificially and starts dividing. This was the technique used to create Dolly the cloned sheep: the nucleus was removed from one of her eggs and replaced by the nucleus of a cell from her mammary gland.
Could one take the egg of a mouse and replace its nucleus with the nucleus of a rat?
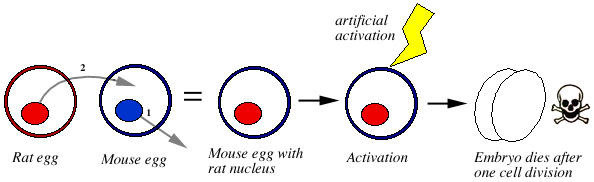
Waksmundzka (1994) transplanted the nuclei of rat eggs into mouse eggs. These developed to the 1- or 2-cell stage, then degenerated (Fig. 4).
|
|
|
Figure 4. The nucleus of a rat's egg is placed into an enucleated mouse egg. The resulting hybrid egg is activated artificially. The hybrid egg may start dividing, but dies after one cell division, at the two cell stage. |
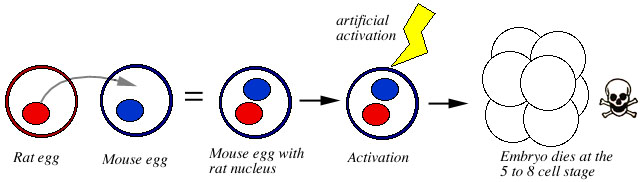
If the mouse nucleus was not removed first, and the rat nucleus was inserted next to it, the embryos made it to the 5- or 8-cell stage, then degenerated. The author concluded that there was a serious incompatibility between the nucleus and cytoplasm of the two species from the very beginning of development.
|
|
|
Figure 5. The nucleus of a rat egg is placed inside a mouse egg. The resulting hybrid embro is activated artificially. The hybrid embryo makes it to the 5 or 8 cell stage, then dies. |
Chimeras: merging two rat and mouse embryos
A chimera is an organism that has genetically different cell populations, arising from more than one fertilized ovum.
Here's how it works: right after fertilization, the new single-cell egg divides into two cells, then divides again into four, then eight. At this early stage the embryo is a microscopic ball of undifferentiated cells called a blastomere. This ball of cells continues dividing, and when it reaches 16 cells it is called a morula. The morula continues dividing, and becomes a sphere of cells around a hollow middle, called a blastocyst. The blastocyst has two types of cells: an outer layer and a little blob of cells stuck to the inside wall (called the inner cell mass). The inner cell mass develops into the embryo itself, the outer layer becomes part of the placenta.
At the blastomere stage, the embryo is very resilient to the addition or subtraction of cells. For example, it can be split in half, with a couple cells in one cluster and a couple cells in another cluster. These two clusters, if implanted into a hormonally primed uterus of the same species, grow up to be two genetically identical individuals. This splitting can happen naturally, which is where identical twins come from.
|
|
|
Figure 6. Chimeric mouse from one tan and one black mouse embryo. Charles River Labs . |
Very rarely, chimeras occur naturally in nature, including in humans (e.g. Fitzgerald et al. 1979, Iselius et al. 1979, Mayr et al. 1979, Repas-Humpe et al. 1999, Strain et al. 1998, Verp et al. 1992).
What about chimeras made between embryos of different species?
Chimeras have been made between the house mouse (Mus musculus) and a very close relative, the Asian mouse (Mus caroli, also known as the ricefield mouse or the Ryukyu mouse). These species do not normally mate, though sterile hybrid offpsring can be produced at a low rate by artificial insemination (Rossant et al. 1983). Chimeric embryos grow and implant successfully in the uterus, are brought to term and born alive, and grow up to be fertile adults. These individuals have roughly equal numbers of cells from the house mouse and the Asian mouse (Konyokhov 1986, Rossant and Frels 1980, Rossant 1985, Rossant and Chapman 1983, Rossant et al. 1983). Successful chimeras have also been produced between sheep and goats (e.g. Fehilly et al. 1984, Polzin et al. 1987, MacLaren et al. 1993)
Can a chimera be produced from a mouse and rat embryo?
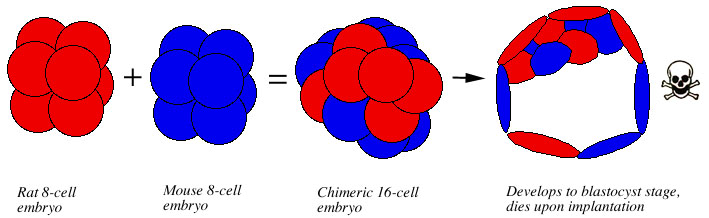
Chimeric embryos between mouse (Mus musculus) and rat (Rattus norvegicus) have been created several times with the aggregation method (Mulnard 1973, Stern 1973, Zeilmaker 1973, Rossant 1976, Tachi and Tachi 1980). Some of embryos developed normally in vitro up to the blastocyst stage. However, when they were implanted into the hormonally primed uteri of mice or rats, they died at the time of implantation or shortly afterwards (Rossant 1976, Tachi and Tachi 1980) (Fig. 7).
|
|
|
Figure 7. Creation of rat-mouse chimeras using the aggregation method. An 8-cell rat embryo and an 8-cell mouse embro are pressed together to form a hybrid 16 cell embryo. The enbryo develops normally in vitro to the blastocyst stage, but dies upon implantation in the uterus of a mouse or rat. |
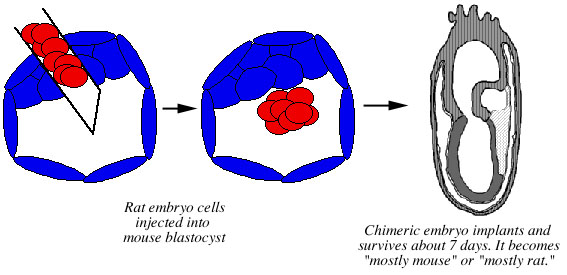
Chimeric embryos have also been created using the injection method. These were implanted and 28 out of 56 of them developed normally up to the total age of 7.5-9.5 days. At later stages of development, the mouse cells appeared to have a selective advantage: they came to dominate the embryo, while rat cells became rare. The embryos were killed and examined 7 days after transfer, and nine of them were found to be chimeras. Four were examined for percentages of mouse vs. rat cells, and three were found to have a predominance of mouse cells (67-97% mouse) while one was found to be primarily rat (78% rat) (Gardner and Johnson 1973) (Fig. 8).
|
|
|
Figure 8. Creation of rat-mouse chimeras using the injection method. Cells from an early rat embryo are injected into the cavity of a mouse blastocyst. The resulting chimeric embryo implants and develops normally for 7.5-9.5 days. One cell lineage came to dominate each embryo: most of the resulting embryos were found to be predominantly mouse, one embryo was found to be predominantly rat. |
Transplants: the secondary chimera
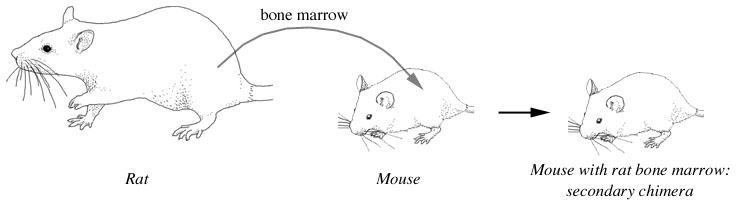
Cells or organs from one species can be injected or transplanted into an animal of another species. The resulting animals have cells that come from different organisms, and are called secondary chimeras.
For example, mice with depressed immune systems have been injected with rat bone marrow (e.g. French et al. 1992, Spach et al. 1995, Balazs et al. 1998), or human bone marrow (Böcher et al. 1999). The bone marrow establishes itself and produces white blood cells normally. A severely immunodepressed mouse will also tolerate the transplant of an organ from another species, such as skin, cartilage, feathers, or a piece of bone.
These mice with rat-tissue transplants have cells from both species, and therefore fit the definition of a chimera, but they are technically not hybrids.
|
|
|
Figure 9. Secondary chimeras are the result of tissue transplants. A mouse with transplanted bone marrow from a rat is a secondary chimera. |
Transgenic rats
A transgenic animal is one that has been modified by having a gene artificially inserted into its genome early in development, such that all its cells contain the new gene and it can pass the new gene onto its offspring (here's how). The new gene may be from the same species as the host, but it is frequently from a different species.
For example, researchers at Nexio Biotechnology inserted the gene for spider silk into a goat egg. The resulting transgenic goat carried the spider silk gene in every one of its cells. The gene was expressed in the milk gland and produced spider silk (Nexia references).
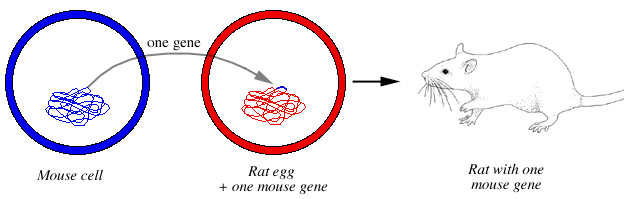
An enormous number of transgenic rat lineages have been created, including transgenic rats that carry mouse genes (Fig. 10). For example, Mullins et al. (1990) created a transgenic rat that carried the mouse Ren-2 gene, and Alhonen et al. (2000) produced a transgenic rat that included the mouse SSAT gene.
|
|
|
Figure 10. Creation of a transgenic rat. A gene from a mouse is inserted into a rat egg. The rat egg develops into a rat that carries one mouse gene. |
Human genes have been inserted into rats as well. For example, Howland et al. (2002) inserted the human SOD1 gene into rats for the study of Lou Gherig's disease. Asamoto et al. (2002) created transgenic rats with a human proto-oncogene.
The number of transgenic lineages is enormous and well beyond the scope of this website. The University of Washington alone produces 50-60 new types of transgenic mice per year. Here's further reading:
- Oak Ridge National Laboratory database of transgenic animal lines
- Transgenic Animal Facility at UW Madison.
- Jackson Lab internet resources for transgenic and targeted mutation research
Hybrid cell lines
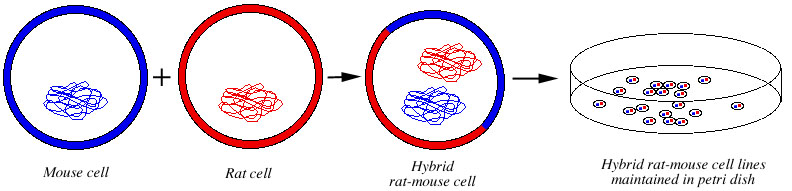
It is possible to create cell lines (populations of cells that live for generations in a petri dish) that contain DNA of different species. These hybrid cell lines are constructed by fusing cells from different species and maintaining the resulting merged cells and their descendants in a culture medium.
Many such hybrid cell lines have been created, such as rat-mouse (e.g. Helou et al. 1997) (Fig. 11), canine-rodent (e.g. Langston et al. 1997), human-cow (van Olphen and Mittal 2002), human-mouse, human-hamster (Geard and Jenkins 1995), rodent-canine (Langston et al. 1997), mink-hamster, hamster-chicken, and so forth. They are kept in cell banks and are frequently used in research.
|
|
|
Figure 11. Creation of hybrid rat-mouse cell lines. The cell of one species is fused with the specially prepared cell of another (an "immortal cell"), producing a merged hybrid cell with sets of DNA from both species. The resulting hybrid cells cannot develop into individuals. They are maintained in petri dishes and are used in research. |
Such hybrid cells sometimes lose the chromosomes from one of the particpating species, or rearrange them or delete parts of them, and studies are conducted to determine which chromosomes have been retained in a particular hybrid cell line (e.g. Helou et al. 1998). This tendency of hybrid cell lines to expunge the chromosomes of one species has been used to map genes onto chromosomes (Ruddle 1972).
So, hybrid cell lines, formed by the fusion of two cells from different species, can be maintained on artificial medium. These cells will not, however, grow up to be a whole hybrid organism.